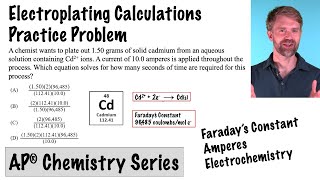
Published On Jul 10, 2023
Delta G (Gibbs Free Energy), Delta H (Enthalpy), and Delta S (Entropy) define whether a reaction will be thermodynamically favorable or thermodynamically unfavorable. These terms are often also referred to as "spontaneous" or "non-spontaneous." This is a multiple choice question (MCQ) that is specifically designed to prepare for the AP Chemistry test, but may also be relevant for other exams and curricula such as A-levels, IGCSE, JEE, NEET, MCAT, DAT, OAT, GRE and SAT.
show more