Published On Jun 16, 2023
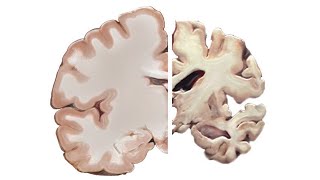
If you live long enough, either you will have Alzheimer’s yourself or it’ll be one of your relatives or one of your friends or… maybe more than one. This is a public health issue of the 21st century which is getting very personal. Impressive failures in all our attempts to figure out the mechanism of the disease and find any disease modifying solutions have been disappointing. Where are we today? What are the new -mabs which are making the headlines? Is this a hype promising billion-dollar earnings to Pharma or is this a hope for disease modification?
Discussants:
Professor Aaron Kesselheim (Harvard Medical School)
Professor Peter Whitehouse (Neurology, Case Western Reserve University)
Professor Frank Harrell (Biostatistics, Vanderbilt University)
Dr Richard Oakley (Alzheimer’s Society, UK)
Comments from chat:
For private sector investors, the US "is" the market! Apologies to my fellow Europeans.
Is the amyloid hypothesis as vague and ill-founded as the "chemical imbalance" theory of depression?
I'll leave it to others to decide if it is "ill-founded", but it certainly is nowhere near a predictive surrogate outcome in my view. Many individuals with dementia have substantial amyloid buildup on imaging; so do many individuals without dementia. Fails the "reasonably likely to predict clinical benefit" test the FDA states for accelerated approval in my view.
Question to Rich Oakley: Some have argued that approval of even clinically marginally effective AD drugs helps to encourage companies to stay in the AD research field - else they might give up on looking for effective treatments and we will never see effective drugs. What is your take on this argument?
At best IMO it represents the first faltering steps in what will be a very long journey in AD treatment-the Wright Bros of neurodegeneration if you will. Because of disease complexity & heterogeneity there will be other targets as well as superior disease classification.
Frank's endpoint / scale / clinical relevance comments strike me as relevant across a wide range of diseases. Are there therapy areas where improved disease measures have made a real difference? Who has the right incentives to break the inertia here? I can understand why companies are reluctant to take "risks" with new scales.
Fascinating! Thanks. I must go and look up that covid work.
Covid work is at https://hbiostat.org/proj/covid19
Should we not be querying that, like cancer, the only way forward is to focus heavily on therapeutic solutions coming at $billion costs? Alternative approaches? Thinking out of the therapeutic box for longer term solutions.
A recent report in Neurology found “accelerated loss of brain volume” in people treated with B-amyloid drugs. Some called this a pseudo-atrophy with no relevant impact on function; others were not so sure. Is it possible these drugs could lead to worse decline in the long run?
In the US, this is an area of huge unmet need in what is essentially the biggest market (Medicare) on the planet. I cannot imagine development slowing down one bit, regardless of the status of the first entrants.
My view, in respect to Till's question, is that commercial R&D investment follows the money. Contrast the "bubble" of cancer R&D chasing high prices and revenues in the cancer market with the dearth of antibiotic investment (low prices plus "stewardship" for low revenues). I get the sense that the approval of Alzheimer's drugs that are marginally effective has made it easier to get private sector money for Alzheimer's R&D. It has become easier to bet other people's pension money on Alzheimer's drugs.
Fascinating talk. Thanks so much to the speakers.
There are a number of drugs in development targeting Tau which may “map” on the timeline of AD better than amyloid.
Thank you great discussion